-

0.2ml PCR Octet Tubes
-

0.2ml PCR Octet Tubes - With Caps
-
96-Well Semi-Skirted & Non-Skirted PCR Plates
-

8-Well Enzyme Strips & Plates
-

96 Magnetic Bar Sleeve
-

8-Link Magnetic Bar Set
-
.jpg?imageView2/2/format/jp2/q/100)
96 Round Bottom Deep Well Plates
-
-1.jpg?imageView2/2/format/jp2/q/100)
96 Cone Bottom Deep Bore Plates
-

14ml 50ml Centrifuge Tubes
-

2ml 1.5ml 0.6ml Microcentrifuge Tubes
Copper death: the latest discovered way of cell death!
Posted by Admin | 28 Jan
Cell death is a normal life phenomenon, and its related research has been a hotspot in the field of life science. Of the different mechanisms of a sentence, the mode of cell death is also different, common cell apoptosis, pyroptosis, necrosis, stick death, and so on. Among them, iron death, a new type of cell death method named in 2012, has become the focus of research in recent years. Similar to iron, copper is also an indispensable trace element in all living organisms, and is usually maintained at extremely low levels in mammalian cells. Intracellular copper ion concentrations above the threshold for maintaining the homeostatic mechanism would also exhibit cytotoxicity.
In March 2022, Science magazine published a scientific paper entitled Copper-induced cell death by targeting lipoylated TCA cycle protein under its cell death theme, and the first author is Peter Tsvetkov, from the Todd R. Golub team of Broad Institute of Harvard and MIT. In this article, the occurrence mechanism in the researchers' handout is clearly different from the known controlled cell death mode of cell apoptosis, pyroptosis, necrotic apoptosis, and iron death, named "copper death" (Cuproptosis).
Through the analysis of phenomena, mechanisms, and disease models, the researchers found that copper death occurs through the direct binding of copper to the lipo-acylated components of the tricarboxylic acid cycle (TCA). This leads to the aggregation of lipoylated proteins and loss of iron-sulfur cluster proteins, which triggers proteotoxic stress and ultimately cell death.
The researchers first tested 489 different cell lines with different structures of copper ionophore and demonstrated that copper ionophore can induce cell death, which mainly depends on intracellular copper accumulation. To verify whether this mode of death is affected by known modes of cell death, the researchers treated cells by knocking down BAXhe BAK1, a key factor of cell apoptosis, and using known inhibitors of cell death mode (caspase inhibitors of apoptosis, iron death Ferrostatin-1, necro stain-1 to necrotizing apoptosis, and N-pancreatic cysteine in response to oxidative stress), and found that copper ionophore-induced cell death was not eliminated. This suggests that cell death by copper ionophore is a distinct mechanism from the known mode of cell death.
Meanwhile, the researchers observed that cells, which are more dependent on mitochondrial respiration, were about 1,000 times more sensitive to copper ion inducers than to glycolytic-dependent cells. Treatment with mitochondrial antioxidants, fatty acids, and mitochondrial functional agents can significantly change the sensitivity of cells to copper ions.
In addition, electron transport chain (ETC) complex inhibitors, as well as inhibition of mitochondrial pyruvate uptake, reduced copper-induced cell death, none of which had an effect on iron death. At the same time, it was found that the amount of tricarboxylic acid cycle (TCA) related metabolites changed in cells treated with the copper carrier, indicating that cell death may act in the tricarboxylic acid cycle (TCA) stage.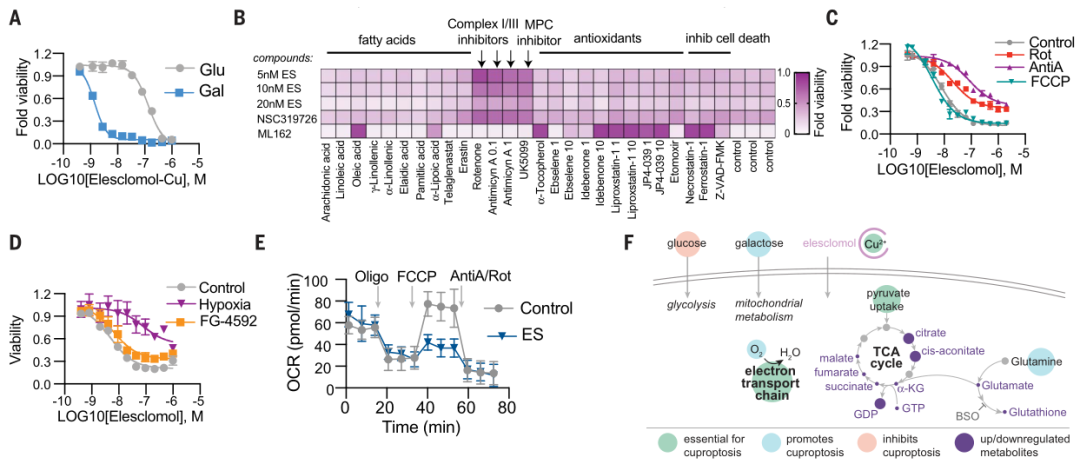
To further explore the metabolic pathways of copper death, a genome-wide CRISPR-Case9 loss-of-function screen identified seven genes associated with copper ionophore-induced cell death, including FDX 1. Studies confirmed that FDX 1 and protein lipoylation when key factors of copper ionophore-induced cell death. The excess of copper promotes the loss of FDEX 1 of lipoylated proteins, leading to the complete loss of protein lipoylation function, and the accumulation of intracellular pyruvate, A-cupro glutarate, and the consumption of succinate indicating that the loss of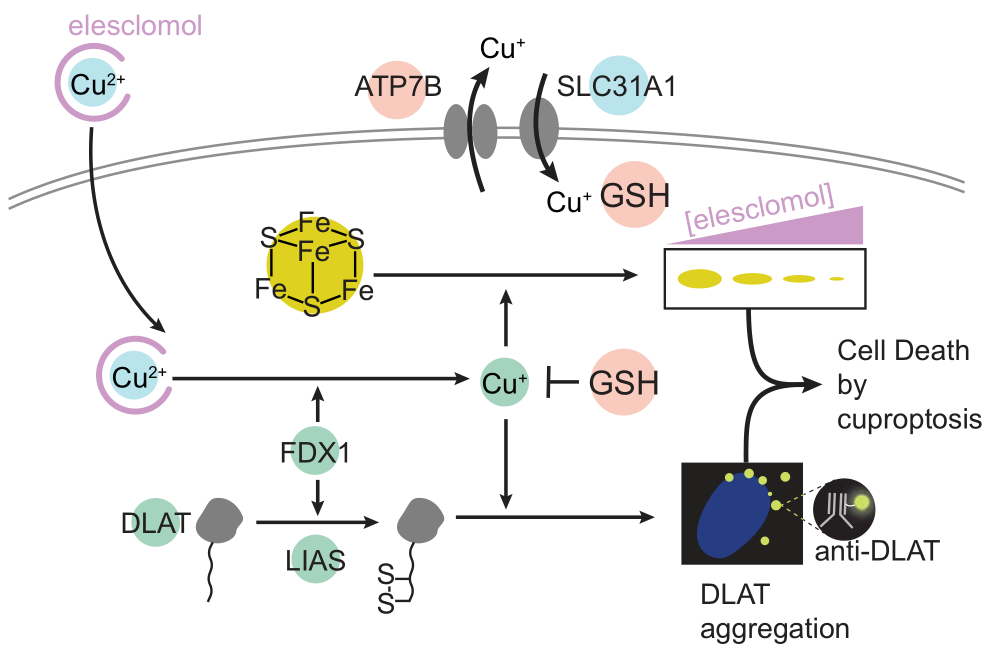
Overall, the team discovered a new type of cell death method and named it copper death (Cuproptosis) to distinguish it from existing cell death methods. The main process of copper death depends on the accumulation of intracellular copper ions, which directly bind the lipoylated components of the tricarboxylic acid cycle (TCA) cycle, leading to the aggregation and deregulation of these proteins, blocking the tricarboxylic acid (TCA) cycle, triggering proteotoxic stress, and inducing cell death. The team further revealed that FDX 1 is a key regulator of copper death and an upstream regulator of protein acylation.
The abundance of FDX 1 and lipoylated proteins, is highly associated with a variety of human tumors. Cell lines with high levels of lipoylated proteins were confirmed to be more sensitive to copper death. These findings suggest that copper ionophore may be a potential therapeutic for cancer cells with such metabolic features.



 English
English 简体中文
简体中文




